Solubility
Solubility is one of the most important physico-chemical properties of a drug/drug candidate. The knowledge of solubility is required from the earliest stages of drug discovery to the latest stage of drug formulation.
Solubility is defined as the maximum amount of a substance that will dissolve in a given amount of solvent at a specified temperature.
Solubility Table
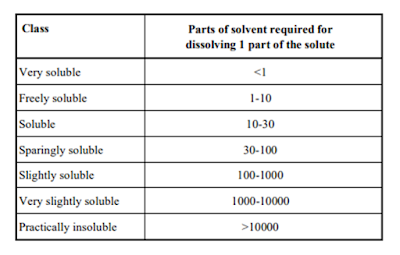
The United States Pharmacopeia (USP) classified the solubility of drugs in seven classes as listed in below table -
Methods of Estimating Aqueous Solubility
The most common aqueous solubility prediction method is General solubility equation (GSE) developed by Yalkowsky and his co-workers expressed as:
In which Sw is the aqueous solubility of drug, mp is the melting point and logKow the logarithm of partition coefficient, the temperature of the water is 25°C.
The GSE indicates that the aqueous solubility will be reduced for compounds with a higher melting point and compounds with a higher tendency to partition into an oil phase (octanol). The logarithm of the octanol–water partition in the GSE accounts for the difference between an ideal solution and an aqueous solution due to the enthalpy of mixing. The GSE can also be used to predict the solubility of ionizable compounds by combining it with the Henderson–Hasselbalch equation if the pKa is known.
Use of GSE requires the measurement of the melting point and partition coefficient (and pKa for ionizable compounds). There are several computer programs that will support the estimation of the partition coefficient and the pKa for compounds based on structure, but this is not the case for melting points. Efforts to develop computational methods to predict aqueous solubilities have relied on training sets of molecules to search for correlations with properties that can be more easily predicted from the structure (e.g., molecular weight, solvent-accessible surface area, number of rotatable bonds, etc.).
The success of these computational approaches is often limited to molecules that are similar to the training set. These calculational methods are adequate for providing assistance in prescreening synthetic candidates, but are not sufficiently accurate to substitute for experimental solubility.
Factors that affect solubility and solubility measurements
- Effect of pH
- Effect of salts and counter-ions
- Effect of co-solvents
- Effect of surfactants
- Effect of complexing agents
- Effect of surface area (Dissolution rate)
- Effect of surface energy (Nanoparticles)

Post a Comment