Residual solvents in pharmaceuticals are defined as organic volatile chemicals that are used or produced in the manufacturing of drug substances, excipients, or dietary ingredients, or in the preparation of drug products or dietary supplement products.
As residual solvents do not provide therapeutic benefit, they should be removed, to the extent possible, to meet safety-based limits, ingredient and product specifications, good manufacturing practices, or other quality-based requirements.
Classification of Residual Solvents
- Class 1 (solvents to be avoided)
- Class 2 (solvents to be limited)
- Class 3 (solvents with low toxic potential)
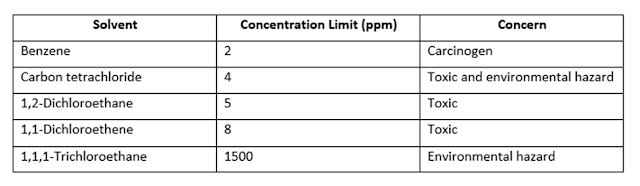
Control Limits for Class 1 Residual Solvents in Official Products: Solvents to Be Avoided
Control Limits for Class 2: Solvents to Be Limited
Class 3: Solvents with Low Toxic Potential
OPTIONS FOR DESCRIBING LIMITS OF CLASS 2 AND CLASS 3 RESIDUAL SOLVENTS
Option 1—Concentration Limit
The concentration limits in ppm stated in Table 3 for Class 2 solvents and the general requirement for Class 3 (5000 ppm, equivalent to 0.5% w/w) are used. The values for Class 2 solvents were calculated using the equation below by assuming a product weight of 10 g administered daily.
Concentration (ppm) = (1000 µg/mg × PDE)/dose
Here, PDE is given in terms of milligrams per day (mg/day), and dose is given in grams per day (g/day). These limits are considered acceptable for all drug substances, excipients, dietary ingredients, and official products.
Therefore, Option 1 may be applied if the daily amount is not known or does not exceed 10 g. If all official substances (drug substances, excipients, and/or dietary ingredients) in a formulation or dietary supplement meet the limits given in Option 1, these components may be used in any proportions. No further calculation is necessary, provided that the daily amount does not exceed 10 g. Products that are administered in doses (daily intake for dietary supplements) greater than 10 g/day are to be considered under Option 2.
Option 2—Summation of Components Content
Option 2 uses the PDE and the actual maximum daily amount of the product to calculate solvent exposure and assess compliance.
Option 2 must be used to demonstrate compliance with this chapter where the maximum daily dose of the official product exceeds 10 g/day or where at least one component in the formulation exceeds the Option 1 limits.
These limits are applied by summing the amounts of a residual solvent present in each of the components of the official product. The contribution of each solvent per day should result in a total amount that does not exceed the PDE.
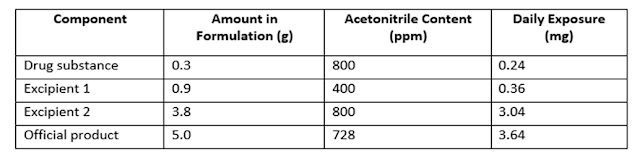
Consider the example of the application of Option 1 and Option 2 limits to acetonitrile concentration in an official product. The PDE for acetonitrile is 4.1 mg/day, thus the Option 1 limit is 410 ppm. The maximum administered daily mass of an official product is 5.0 g, and the official product contains two excipients. Assuming that there is no other source of acetonitrile in the manufacturing process, the calculated official product content of acetonitrile and the daily exposure are given in below table –
Table: Example of Meeting the Requirement for Acetonitrile as per Option 2
Excipient 1 meets the Option 1 limit, but the official substance, Excipient 2, and Official product do not meet the Option 1 limit. Nevertheless, the product meets the Option 2 limit of 4.1 mg/day and thus conforms to the recommendations in above.
Read also:



إرسال تعليق